Gas leakage involves the release of gas from a containment system, whether it’s a pipe, or vessel. From a thermodynamic perspective, the process of gas leak can be understood through principles such as ideal gas law, thermodynamic equilibrium, and entropy (s).
Gas Thermodynamic Process

Isentropic (Adiabatic) Process
- An isentropic process is one in which the entropy (s) of a system remains constant. In fact, gas leakage trends to increase entropy as the gas expands into a larger volume, resulting in a more disordered state since the entropy (s) is a measure or the disorder or randomness of a system.
- In practical terms, an isentropic process is often an idealization of a process that occurs without any heat exchange with the surrounding environment. (Q = 0)
- For an ideal gas undergoing an isentropic process, the relationship between pressure (P) and Volume (V) can be described as following;
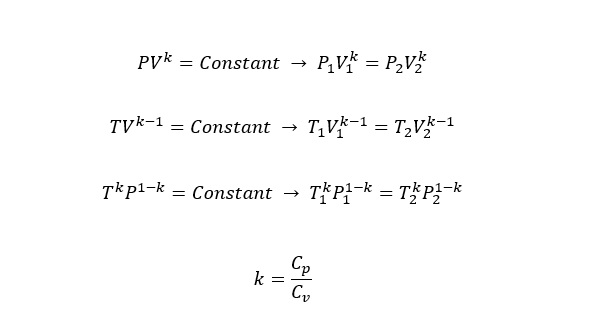
- For example, the isentropic process is the expansion of gas in a piston-cylinder.
Isothermal Process
- An isothermal process is one that occurs at constant temperature.
- In an isothermal process, the internal energy of the system remains constant (dU = 0), meaning that any heat added to or removed from the system is entirely converted into work (Q = -W)
- For an ideal gas undergoing an isothermal process, the relationship between pressure (P) and volume (V) is described by the ideal gas law.
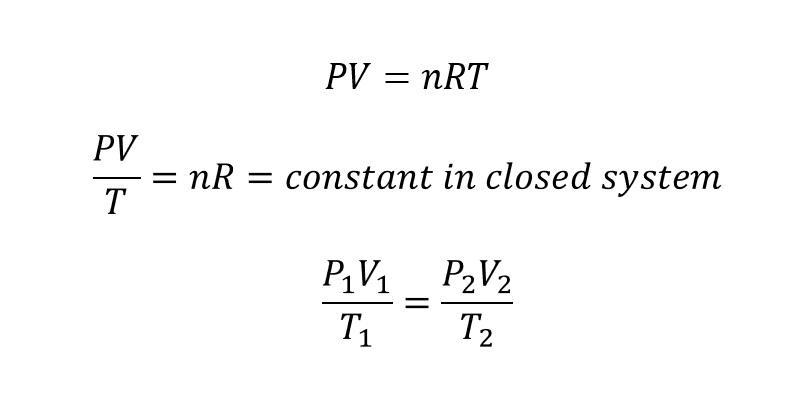
- The example process of isothermal is a gas confined within a cylinder fitted with a movable piston. And to maintain the temperature, this cylinder shall be placed in a large water bath with provided temperature by heat sink.

And the gas leakage formula is presented in below.

When apply the discharge coefficient (CD)
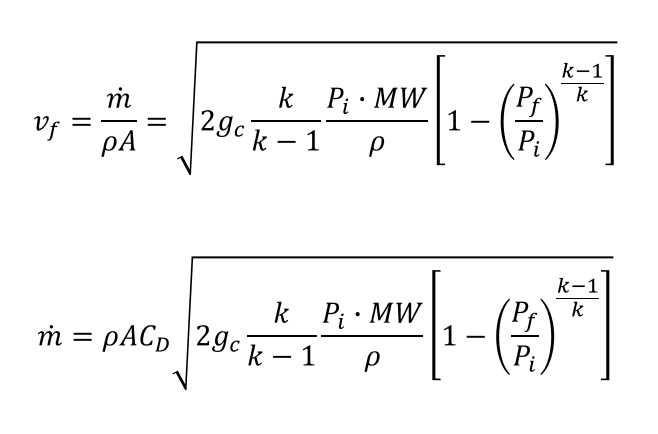
- Sharp-edged orifice and Reynold number > 30,000: CD = 0.61
- Short section of pipe attached to a vessel and L/D ratio > 3: CD = 0.81
- Conservative approach: CD = 1